Flutivue 0.05% Nasal Spray 10 ml
MRP ₹225
(Inclusive of all Taxes)
₹33.8 Cashback (15%)
Know Your Delivery Time
Provide Delivery Location

Secure Payment

India's Most Trusted Pharmacy

Genuine Products
Composition :
Manufacturer/Marketer :
Consume Type :
Return Policy :
Expires on or after :
About Flutivue 0.05% Nasal Spray 10 ml
Flutivue 0.05% Nasal Spray 10 ml is indicated for managing seasonal allergic symptoms, and perennial allergic and non-allergic rhinitis in adult and paediatric patients aged four years and older. Seasonal allergies are caused by exposure to airborne substances (such as pollens) that are only present at specific seasons of the year. Perennial allergies are caused by year-round indoor exposure to airborne pollutants (such as house dust). Perennial non-allergic rhinitis, also known as vasomotor rhinitis, is a chronic illness characterised by symptoms similar to allergic rhinitis (runny nose, stuffy nose, sneezing, and nasal itching) but lasting for more than nine months each year.
Flutivue 0.05% Nasal Spray 10 ml contains fluticasone propionate. Fluticasone propionate belongs to the class of corticosteroids that work by inhibiting the release of certain chemicals in the body that cause inflammatory reactions. Thereby, it provides relief from a blocked or runny nose, sneezing, itching, watery eyes and an itchy nose.
Flutivue 0.05% Nasal Spray 10 ml is available on prescription only. Flutivue 0.05% Nasal Spray 10 ml must be taken as directed by the healthcare professional or as per leaflet information to get the maximum benefit of Flutivue 0.05% Nasal Spray 10 ml. As very little medicine of Flutivue 0.05% Nasal Spray 10 ml is absorbed into the rest of the body, it's not likely to give you side effects. In rare cases, you may experience headaches, nausea, vomiting, sore throat, nose bleeds, trouble breathing, cough, burning, or itching. Most of these side effects do not require medical attention and gradually resolve over time. However, if the side effects persist or worsen, please consult your doctor.
Avoid using if you have a known allergy or hypersensitive reaction to fluticasone Propionate. If you are a pregnant or nursing mother, do not use Flutivue 0.05% Nasal Spray 10 ml without first consulting your doctor. Avoid Flutivue 0.05% Nasal Spray 10 ml use in patients with recent nasal ulcers, surgery, or trauma. It is not recommended for use in children under four years of age. Children aged four years and older can use Flutivue 0.05% Nasal Spray 10 ml under the supervision of a healthcare professional. The development of eye conditions such as cataracts and glaucoma has been reported with the use of fluticasone nasal sprays. You should have a yearly eye exam to check for these conditions. Before using Flutivue 0.05% Nasal Spray 10 ml, inform your doctor if you have or have ever been treated for fungal, bacterial, viral or parasitic infections. Also, tell your doctor if you have tuberculosis (a type of lung infection) before taking this medicine.
Uses of Flutivue 0.05% Nasal Spray 10 ml
Flutivue 0.05% Nasal Spray 10 ml is a corticosteroid medicine used to relieve inflammation and allergy-related nasal symptoms. The detailed uses of Flutivue 0.05% Nasal Spray 10 ml are as follows:
- Seasonal allergic rhinitis: Flutivue 0.05% Nasal Spray 10 ml reduces sneezing, nasal congestion, and runny nose caused by seasonal allergens like pollen.
- Perennial allergic rhinitis: Flutivue 0.05% Nasal Spray 10 ml is used to treat allergy symptoms that occur due to dust, mold, animal allergies, or other indoor triggers.
- Perennial nonallergic rhinitis: Flutivue 0.05% Nasal Spray 10 ml reduces nasal inflammation and symptoms unrelated to allergens, such as those induced by environmental irritants.
- Prevention of allergy flare-ups: Flutivue 0.05% Nasal Spray 10 ml is also used regularly during known allergy seasons to prevent symptom development.

Have a query?
Directions for Use
- It is usually used once daily; however, follow your doctor’s instructions on the dosage and timing of this medication for effective results.
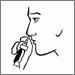
- Insert the tip of the bottle into a nostril while closing the other nostril and spray towards the sides of the nostril. Keep your head straight and breathe gently. Repeat the same process for another nostril if needed.
- Before using the nasal spray, clean your nose by gently blowing over a handkerchief or tissue.
Medicinal Benefits
Flutivue 0.05% Nasal Spray 10 ml contains fluticasone propionate. Fluticasone propionate belongs to the class of corticosteroids that work by inhibiting the release of certain chemicals in the body that cause inflammatory reactions. As a result, it relieves symptoms such as a blocked or runny nose, sneezing, itching, watery eyes, and an itchy nose.
How Flutivue 0.05% Nasal Spray 10 ml Works
Storage
- Inform your doctor about the symptoms you're experiencing due to medication.
- Your doctor may adjust your treatment plan, which could include changing your medication, adding new medications, or offering advice on managing your symptoms.
- Practice good hygiene, including frequent handwashing, avoiding close contact with others, and avoiding sharing utensils or personal items.
- Stay hydrated by drinking plenty of fluids to help loosen and clear mucus from your nose, throat, and airways.
- Get plenty of rest and engage in stress-reducing activities to help your body recover. If your symptoms don't subside or worsen, consult your doctor for further guidance.
- A blocked nose can be relieved by drinking more water, which helps clear fluids.
- Use saline nasal spray available over the counter to relieve blockage or blow harder to remove the mucus.
- Use nasal strips that can be placed on the nose to widen nostrils and increase airflow.
- Keep a humidifier around to moisten air at home/workplace.
- Throat pain can worsen if there is no proper rest for your throat.
- Drink plenty of warm fluids and frequently gargle with salt water.
- Humidify the surrounding air using a humidifier, as dry air may increase dryness and throat pain.
- Consider taking lozenges or hard candy throat pain relievers, which give a soothing effect.
- Take enough rest and stay in warm places to reduce pain quickly.
- Tell your doctor about the cough symptoms you're experiencing, which may be triggered by your medication.
- Your doctor may adjust your treatment plan by changing your medication, adding new medications, or providing guidance on managing your cough symptoms.
- Practice good hygiene, including frequent handwashing, avoiding close contact with others, and avoiding sharing utensils or personal items.
- Stay hydrated by drinking plenty of fluids, such as water, tea, or soup, to help thin out mucus and soothe your throat.
- Get plenty of rest and engage in stress-reducing activities to help your body recover. If your cough persists or worsens, consult your doctor for further guidance.
- Get plenty of rest and avoid activities that tire you out to allow your body fight the infection.
- Drink lots of fluids to help loosen mucus in the lungs.
- Use over-the-counter medications like paracetamol or ibuprofen to manage fever.
- Use a humidifier as it helps soothe irritated airways.
- Do not make changes to your medication schedule or take over-the-counter medicines without consulting your doctor.
- To keep your vocal cords lubricated and hydrated, drink lots of water.
- Suck on ice chips, popsicles, or lozenges to clear your throat and reduce hoarseness.
- Your voice cords may be soothed and healed by honey's antibacterial and anti-inflammatory properties.
- Gargle with salt water.
- Using a steam humidifier or inhaling steam might help minimize hoarseness and release mucus.
- If you experience symptoms like coughing, wheezing, chest tightness, or difficulty breathing after taking medication, seek medical attention immediately.
- Your healthcare provider will work with you to stop the medication causing the reaction, start alternative treatments, and provide supportive therapy.
- To manage symptoms and prevent complications, follow your doctor's advice to use inhalers or nebulizers as prescribed, practice good hygiene, avoid irritants, stay hydrated, and get plenty of rest.
- Regularly track your symptoms and report any changes or concerns to your healthcare provider.
What if I have taken an overdose of Flutivue 0.05% Nasal Spray 10 ml
Drug Warnings
Flutivue 0.05% Nasal Spray 10 ml is not suitable for some people. Avoid using Flutivue 0.05% Nasal Spray 10 ml if have a known allergy or hypersensitive reaction to fluticasone Propionate. If you are a pregnant or a nursing mother, do not consume Flutivue 0.05% Nasal Spray 10 ml without first consulting your doctor, as it will only be prescribed if the benefits overweight the risks. Patients with recent nasal ulcers, surgery, or trauma should avoid using this product. The development of eye conditions such as cataracts and glaucoma has been reported with the use of fluticasone nasal sprays. You should have a yearly eye exam to check for these conditions. Before using Flutivue 0.05% Nasal Spray 10 ml, please tell your doctor if you have or have ever been treated for fungal, bacterial, viral or parasitic infections. Also, tell your doctor if you have tuberculosis (a type of lung infection). The safety and effectiveness in children under the age of four have not been satisfactorily proven. Children aged four years and older can use Flutivue 0.05% Nasal Spray 10 ml under the supervision of a healthcare professional.
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Co-administration of Flutivue 0.05% Nasal Spray 10 ml with Mifepristone may make Flutivue 0.05% Nasal Spray 10 ml less effective as a therapy.
How to manage the interaction:
Taking Flutivue 0.05% Nasal Spray 10 ml with Mifepristone is not recommended, consult a doctor before taking it. Do not stop using any medications without talking to a doctor.
Co-administration of Flutivue 0.05% Nasal Spray 10 ml with Voriconazole may increase the absorption of Flutivue 0.05% Nasal Spray 10 ml into the bloodstream and increase the risk of side effects.
How to manage the interaction:
Although Flutivue 0.05% Nasal Spray 10 ml and Voriconazole can cause an interaction, they can be taken if prescribed by a doctor. However, if you experience swelling, muscle weakness, excessive growth of facial or body hair contact a doctor. Do not discontinue the medication without consulting a doctor.
Co-administration of Cladribine with Flutivue 0.05% Nasal Spray 10 ml can increase the risk of developing serious infections.
How to manage the interaction:
Although taking Flutivue 0.05% Nasal Spray 10 ml and Cladribine together can evidently cause an interaction, it can be taken if a doctor has suggested it. However, if you experience fever, chills, diarrhea, sore throat, muscle pains, breathing difficulty, blood in your coughing fluid, weight loss, red or irritated skin, body sores, and discomfort or burning sensation when you urinate, consult a doctor. Do not stop using any medications without a doctor's advice.
Coadministration of Itraconazole and Flutivue 0.05% Nasal Spray 10 ml may significantly increase the absorption of Flutivue 0.05% Nasal Spray 10 ml into the bloodstream.
How to manage the interaction:
Flutivue 0.05% Nasal Spray 10 ml and itraconazole may interact, but if a doctor prescribes them, you can still use them. Swelling, high blood pressure, high blood sugar, muscle weakness, depression, acne, stretch marks, easy bruising, bone density loss, cataracts, irregular menstruation, and excessive face or body hair growth are all signs that you need to consult a doctor. Never discontinue taking a medication without consulting a doctor.
Co-administration of Boceprevir with Flutivue 0.05% Nasal Spray 10 ml may significantly increase the absorption of Flutivue 0.05% Nasal Spray 10 ml into the bloodstream.
How to manage the interaction:
Co-administration of Boceprevir and Flutivue 0.05% Nasal Spray 10 ml can lead to an interaction, it can be taken if advised by a doctor. However, if you experience any symptoms like swelling, weight gain, high blood pressure, muscle weakness, depression, acne, thinning skin, stretch marks, easy bruising, bone density loss, menstrual irregularities, excessive growth of facial or body hair, and abnormal distribution of body fat, especially in the face, neck, back, and waist, consult a doctor immediately. Do not stop using any medications without a doctor's advice.
When Conivaptan is taken with Flutivue 0.05% Nasal Spray 10 ml, it can make Conivaptan break down faster in the body.
How to manage the interaction:
Co-administration of Conivaptan and Flutivue 0.05% Nasal Spray 10 ml can lead to an interaction, it can be taken if advised by a doctor. However, if you experience any symptoms like swelling, weight gain, high blood pressure, high blood glucose, muscle weakness, depression, acne, thinning skin, stretch marks, easy bruising, bone density loss, menstrual irregularities, excessive growth of facial or body hair, and abnormal distribution of body fat, especially in the face, neck, back, and waist, consult a doctor immediately. Do not stop using any medications without a doctor's advice.
Co-administration of Desmopressin with Flutivue 0.05% Nasal Spray 10 ml may increase the risk of hyponatremia (low levels of salt in the blood).
How to manage the interaction:
If you have to use Desmopressin and Flutivue 0.05% Nasal Spray 10 ml together, a doctor may adjust the dose or monitor you more frequently to safely use both medications. However, if you experience loss of appetite, headache, nausea, vomiting, lethargy (very tired), irritability, difficulty concentrating, weakness, unsteadiness, memory impairment, confusion, muscle spasm, decreased urination, and/or sudden weight gain, contact a doctor immediately as these may be symptoms of water intoxication (water poisoning) and hyponatremia (low levels of salt in the blood). Do not discontinue the medication without consulting a doctor.
Co-administration of Flutivue 0.05% Nasal Spray 10 ml with posaconazole may increase the level of Flutivue 0.05% Nasal Spray 10 ml in the blood and increase the risk of side effects.
How to manage the interaction:
Although there is an interaction between Flutivue 0.05% Nasal Spray 10 ml and posaconazole, they can be taken together if prescribed by a doctor. However, if you experience muscle weakness, acne, thinning skin, easy bruising, stretch marks, menstrual irregularities, excessive growth of facial or body hair, and abnormal distribution of body fat, especially in the face, neck, back, and waist, contact a doctor. Do not discontinue the medication without consulting a doctor.
Co-administration of Delavirdine and Flutivue 0.05% Nasal Spray 10 ml may significantly increase the absorption of Flutivue 0.05% Nasal Spray 10 ml into the bloodstream.
How to manage the interaction:
Co-administration of Delavirdine and Flutivue 0.05% Nasal Spray 10 ml can lead to an interaction, it can be taken if advised by a doctor. However, if you experience any symptoms like swelling, weight gain, high blood pressure, muscle weakness, depression, acne, thinning skin, stretch marks, easy bruising, bone density loss, menstrual irregularities, excessive growth of facial or body hair, and abnormal distribution of body fat, especially in the face, neck, back, and waist, consult a doctor immediately. Do not stop using any medications without a doctor's advice.
Co-administration of Flutivue 0.05% Nasal Spray 10 ml and Ritonavir may increase the blood levels of Flutivue 0.05% Nasal Spray 10 ml.
How to manage the interaction:
If you are supposed to take Flutivue 0.05% Nasal Spray 10 ml and Ritonavir together, your doctor may adjust the dose or monitor you more frequently to use both medications safely. However, if you experience side effects such as palpitations, swelling, high blood sugar, muscle weakness, acne, depression, thinning skin, easy bruising, stretch marks, bone density loss, cataracts, menstrual irregularities, excessive growth of facial or body hair, and abnormal distribution of body fat, contact a doctor. Do not discontinue the medication without consulting a doctor.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Diet & Lifestyle Advise
- Keep your hygiene in check and your surroundings neat.
- Include ginger in your daily intake. Some anti-inflammatory chemicals in ginger help relax airway membranes.
- Keeping hydrated. Coughing, runny nose, and sneezing can be relieved by drinking beverages at room temperature.
- Stress weakens the immune system and increases the likelihood of being ill. An individual can exercise regularly, meditate, practise deep breathing, and try progressive muscle relaxation techniques to relieve stress.
- Wish for 7-9 hours of sleep per night to keep fit and safe.
- It is best to avoid coming into touch with recognised allergens (allergy-causing agents) such as pollen, dust, etc.
- Avoid certain food that is known to trigger allergies in you.
- Processed foods and those high in sugar and fat should be avoided because they may trigger inflammation.
- Reduce your daily salt intake and replace it with herbs or spices such as garlic, ginger, and turmeric, which contain natural anti-inflammatory ingredients.
- Please avoid contact with dust as it might worsen your symptoms.
- Stop smoking; quitting smoking can significantly reduce the severity and frequency of your symptoms.
- Also, please avoid contact with pollen in the air as it might worsen your symptoms.
Habit Forming
Therapeutic Class
All Substitutes & Brand Comparisons
RX
Out of StockFluvent Nasal Spray
₹259
(₹2.33/ 1MDI)
14% COSTLIERRX
Ezicas Nasal Spray 12 ml
Intas Pharmaceuticals Ltd
₹411
(₹3.08/ 1MDI)
51% COSTLIERRX
Flosooth Nasal Spray 100 MDI
Morepen Laboratories Ltd
₹355
(₹3.2/ 1MDI)
57% COSTLIER
Alcohol
Caution
No interaction is found with Flutivue 0.05% Nasal Spray 10 ml. But as a precautionary measure, do not consume in excessive amounts.
Pregnancy
Caution
The safety of Flutivue 0.05% Nasal Spray 10 ml in pregnant women is unknown. If you are pregnant, consult your doctor before using Flutivue 0.05% Nasal Spray 10 ml. Your doctor may prescribe this medicine only if the benefits outweigh the risks.
Breast Feeding
Caution
The safety of Flutivue 0.05% Nasal Spray 10 ml in breastfeeding is unknown. If you are a breastfeeding mother, consult your doctor before using Flutivue 0.05% Nasal Spray 10 ml. Your doctor may prescribe this medicine only if the benefits outweigh the risks.
Driving
Safe if prescribed
Flutivue 0.05% Nasal Spray 10 ml usually does not affect your ability to drive or operate machinery.
Liver
Caution
Flutivue 0.05% Nasal Spray 10 ml to be used with caution for patients with hepatic disease. Patients should be closely monitored.
Kidney
Caution
Limited information was available for the use of Flutivue 0.05% Nasal Spray 10 ml in patients suffering from kidney impairment. Please consult your doctor if you have any concerns regarding using Flutivue 0.05% Nasal Spray 10 ml in case of kidney impairment. Your doctor will prescribe only if the benefits outweigh the risks.
Children
Caution
The safety and effectiveness of Flutivue 0.05% Nasal Spray 10 ml in children under the age of four have not been established. Children aged four years and older can use Flutivue 0.05% Nasal Spray 10 ml under the supervision of a healthcare professional.
Heart
Inform your doctor if you have heart disease before using Flutivue 0.05% Nasal Spray 10 ml. Your doctor will prescribe only if the benefits outweigh the risks.
Geriatrics
Caution
Flutivue 0.05% Nasal Spray 10 ml should be used with caution in elderly patients due to an increased risk of side effects. It is important to inform a healthcare professional before use.
FAQs
Flutivue 0.05% Nasal Spray 10 ml is indicated for managing seasonal allergic symptoms and perennial allergic and non-allergic rhinitis.
Fluticasone propionate is present in Flutivue 0.05% Nasal Spray 10 ml. Fluticasone propionate is a corticosteroid that prevents the release of specific molecules in the body that trigger inflammatory responses. As a result, it relieves symptoms such as a blocked or runny nose, sneezing, itching, watery eyes, and an itchy nose.
Avoid use in patients with recent nasal ulcers, surgery, or trauma. Please check with the doctor if you have concerns regarding this.
It may take several days of regular use to improve your condition. If your symptoms do not improve or get worse, consult your healthcare provider.
Do not stop using the Flutivue 0.05% Nasal Spray 10 ml unless your healthcare provider tells you to do so. Suddenly stopping treatment with Flutivue 0.05% Nasal Spray 10 ml can make you feel unwell and may recur your symptoms.
It is not advised to be used in persons with glaucoma or cataracts. The use of nasal corticosteroid medications may aggravate glaucoma and/or cataracts.
Flutivue 0.05% Nasal Spray 10 ml may cause headaches in a few cases. Rest and drinking plenty of fluids are recommended. Don't consume too much alcohol. Consult your doctor if the headache persists or becomes severe.
Flutivue 0.05% Nasal Spray 10 ml is used as and when needed. In case you are on a scheduled dose, take the missed dose as soon as you remember it. However, if it's almost time for the next dose, do not use more quantities to make up for a missed one.
Country of origin
Disclaimer
Author Details
We provide you with authentic, trustworthy and relevant information
Reference
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020121s045lbl.pdf
- https://www.msdmanuals.com/en-in/home/immune-disorders/allergic-reactions-and-other-hypersensitivity-disorders/year-round-
- https://www.msdmanuals.com/en-in/home/immune-disorders/allergic-reactions-and-other-hypersensitivity-disorders/seasonal-allergiesallergies#:~:text=Year%2Dround%20(perennial)%20allergies,allergy%20usually%20suggest%20the%20diagnosis.
- https://lakesurgentcare.com/ufaqs/whats-the-difference-between-seasonal-and-perennial-allergies/#:~:text=Perennial%20allergies%20refer%20to%20specific,mold%2C%20insect%20or%20grass%20growth.
- https://pubmed.ncbi.nlm.nih.gov/14984552/
- https://www.drugs.com/drug-interactions/fluticasone-nasal.html
Buy best Ear, Nose & Oropharynx products by
Entod Pharmaceuticals Ltd
Cipla Ltd
Lincoln Pharmaceuticals Ltd
NuLife Pharmaceuticals
Nri Vision Care India Ltd
Glenmark Pharmaceuticals Ltd
Macleods Pharmaceuticals Ltd
Dr Reddy's Laboratories Ltd
Leeford Healthcare Ltd
Pristine Pearl Pharma Pvt Ltd
Centaur Pharmaceuticals Pvt Ltd
Chethana Pharmaceuticals
Mankind Pharma Pvt Ltd
Zuventus Healthcare Ltd
Zydus Healthcare Ltd
Indoco Remedies Ltd
Intas Pharmaceuticals Ltd
Lupin Ltd
Sun Pharmaceutical Industries Ltd
Troikaa Pharmaceuticals Ltd
Megma Healthcare Pvt Ltd
Zydus Cadila
Auskincare Formualation Pvt Ltd
Dwd Pharmaceuticals Ltd
NVK Pharma
Sapient Laboratories Pvt Ltd
Torque Pharmaceuticals Pvt Ltd
Vilco Laboratories Pvt Ltd
Bell Pharma Pvt Ltd
Blubell Pharma
Capital Pharma
German Remedies Ltd
Incus Pharmaceuticals Pvt Ltd
Kaizen Drugs Pvt Ltd
Kavach 9 Pharma & Research Pvt Ltd
Abbott India Ltd
Alkem Laboratories Ltd
Atopic laboratories Pvt Ltd
Biochem Pharmaceutical Industries Ltd
Delcure Life Sciences Ltd
East India Pharmaceutical Works Ltd
Eris Life Sciences Ltd
GlaxoSmithKline Pharmaceuticals Ltd
Medishri Healthcare Pvt Ltd
Nextgen Healthcare
Optho Remedies Pvt Ltd
Ordain Health Care Global Pvt Ltd
Xseed Pharma
Aver Pharmaceuticals Pvt Ltd
Avilius Neutracare
Biochemix Health Care Pvt Ltd
Biozenesis Healthcare
Cadila Pharmaceuticals Ltd
Clyde Pharmaceutical Pvt Ltd
FDC Ltd
Healthgate Pvt Ltd
Lividus Pharmaceuticals Pvt Ltd
Mdc Pharmaceuticals Pvt Ltd
Medgen Drugs And Laboratories Pvt Ltd
Meridian Enterprises Pvt Ltd
Micro Labs Ltd
Morepen Laboratories Ltd
Novalab Healthcare Pvt Ltd
Ocuris Pharmaceuticals Pvt Ltd
Precept Pharma
Respionix Healthcare Pvt Ltd
Salvador Visiontech Pvt Ltd
Sunways (India) Pvt Ltd
Timon Pharmaceuticals Pvt Ltd
Unison Pharmaceuticals Pvt Ltd
West Coast Pharmaceuticals Pvt Ltd
Zee Laboratories Ltd
Adley Formulations
Ajanta Pharma Ltd
Apace Life Sciences Pvt Ltd
Apex Laboratories Pvt Ltd
Aristo Pharmaceuticals Pvt Ltd
BMW Pharmaco India Pvt Ltd
Bacans Biotech Pvt Ltd
Bio Warriors Pharmaceucticals Pvt Ltd
Casca Remedies Pvt Ltd
Chem Med Pharmaceuticals
Dr Morepen Ltd
Elan Pharmaceuticals Pvt Ltd
Elder Pharmaceuticals Ltd
Elivia Life Sciences Pvt Ltd
Elkos Healthcare Pvt Ltd
Exsiva Pharma Pvt Ltd
Floreat Medica Pvt Ltd
Gene Lifecare
Icpa Health Products Ltd
Ikon Remedies Pvt Ltd
Ipca Laboratories Ltd
Klar Sehen Pvt Ltd
Koye Pharmaceuticals Pvt Ltd
Nvk Pharma
Orn Remedies Pvt Ltd
Rosa Lifesciences
Rowez Life Sciences Pvt Ltd
Siloam Pharmaceuticals Pvt Ltd






