Difizma Capsule (Inhalation Powder) 10's

MRP ₹357
(Inclusive of all Taxes)
₹53.5 Cashback (15%)
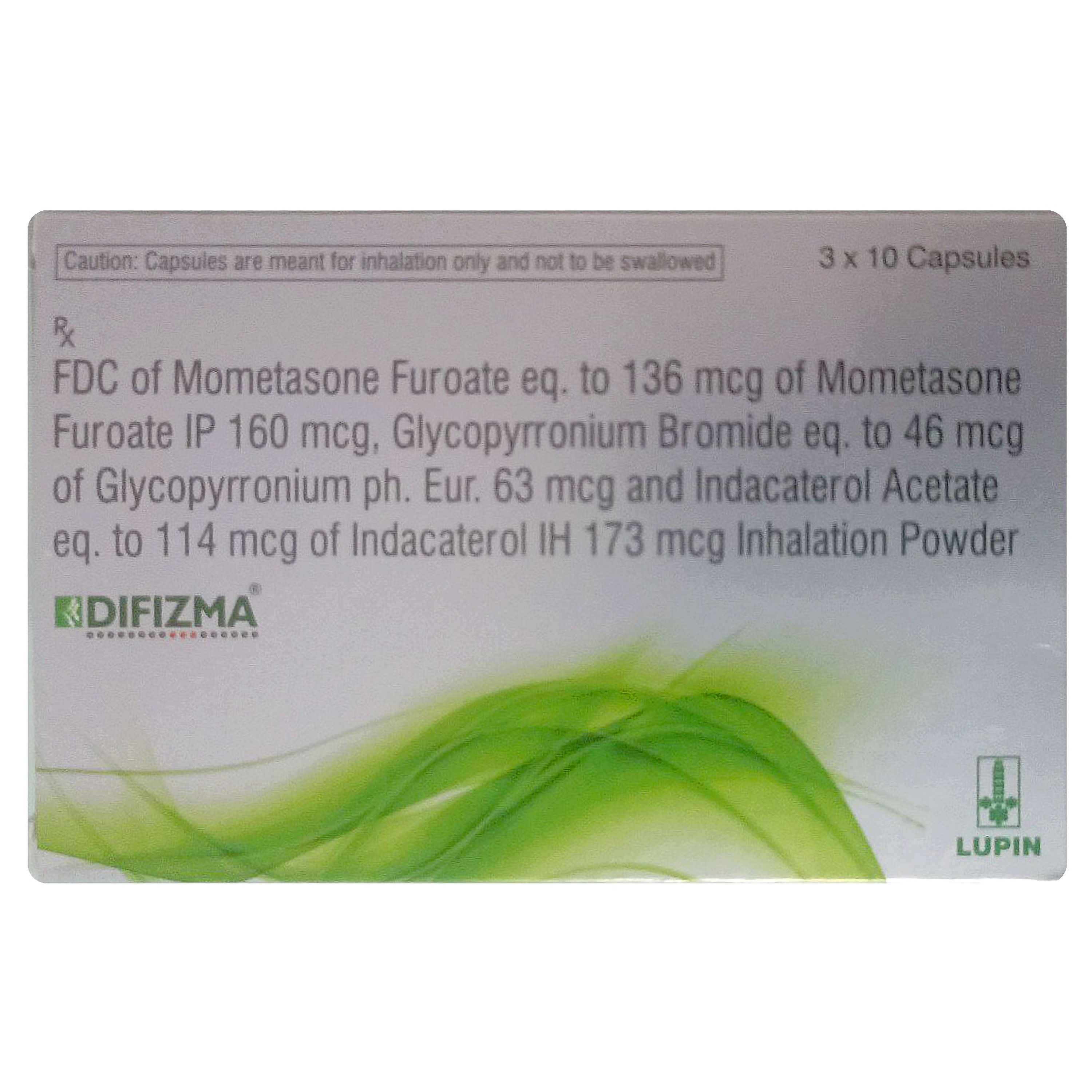
Difizma Capsule (Inhalation Powder) is indicated as a maintenance treatment for asthma in adults. It combines three drugs: Glycopyrronium Bromide and Indacaterol (bronchodilators), and mometasone furoate (corticosteroids). Glycopyrronium Bromide and Indacaterol relax the muscles of the small airways in the lungs, which helps open the airways and makes it easier for air to get in and out. Mometasone Furoate reduces the inflammation (swelling and irritation) in the small airways in the lungs. This gradually eases breathing problems and also helps to prevent asthma attacks.
Know Your Delivery Time
Provide Delivery Location

Secure Payment

India's Most Trusted Pharmacy

Genuine Products
Manufacturer/Marketer :
Consume Type :
Return Policy :
Expires on or after :
About Difizma Capsule (Inhalation Powder)
Difizma Capsule (Inhalation Powder) is used to treat asthma in adults. Asthma is a long-term lung disease that causes airway tightening and inflammation, making breathing difficult.
Difizma Capsule (Inhalation Powder) is a combination of three drugs: Glycopyrronium Bromide, Indacaterol and Mometasone Furoate. Glycopyrronium Bromide and Indacaterol relax airway muscles, making breathing easier. Mometasone Furoate reduces the inflammation (swelling and irritation) in the small airways in the lungs.
In some cases, you may experience certain common side effects such as a runny nose, sore throat, sudden difficulty breathing and chest tightness with coughing or wheezing, oral thrush (a fungal infection in the mouth), headache or fast heartbeat. Talk to your doctor if any of these side effects continue.
If you are allergic to Difizma Capsule (Inhalation Powder) or other medicines, please tell your doctor. Difizma Capsule (Inhalation Powder) is not recommended for children below 18 years. If you are pregnant or breastfeeding, take your doctor’s advice before using Difizma Capsule (Inhalation Powder) .
Uses of Difizma Capsule (Inhalation Powder)
Difizma Capsule (Inhalation Powder) is used in the treatment of Asthma. The detailed uses of Difizma Capsule (Inhalation Powder) are as follows:
- Improve lung function: Difizma Capsule (Inhalation Powder) reduces airway secretions and helps keep the airways open, improving lung function.
- Reduce asthma symptoms: Difizma Capsule (Inhalation Powder) reduces asthma symptoms like wheezing, coughing, and breathlessness.
- Decrease the frequency of asthma attacks: Difizma Capsule (Inhalation Powder) reduces inflammation in the lungs, easing breathing and preventing asthma flare-ups.

Have a query?
Directions for Use
- Difizma Capsule (Inhalation Powder) is recommended to be inhaled once daily at the same time each day or as advised by your doctor.
- Check the label for instructions before using this medication.
- It is for inhalation only. Do not swallow the capsule.
- Remove one capsule from the package and place it into the inhaler device. Close the inhaler and pierce the capsule by twisting or clicking the device.
- Place the mouthpiece into your mouth and seal your lips around it. Inhale deeply and slowly through the mouthpiece, hold your breath for 2-3 seconds, and then exhale slowly.
- Rinse your mouth with water and spit it out after using Difizma Capsule (Inhalation Powder) to avoid fungal infections in the mouth and throat.
Medicinal Benefits
- Difizma Capsule (Inhalation Powder) belongs to the class of respiratory stimulants.
- It contains Glycopyrronium Bromide, Indacaterol and Mometasone Furoate.
- Difizma Capsule (Inhalation Powder) is used as a maintenance treatment of asthma in adults not adequately controlled with a maintenance combination of a long-acting beta2-agonist and a high dose of an inhaled corticosteroid who experienced one or more asthma flare-ups in the previous year.
- Difizma Capsule (Inhalation Powder) relaxes the muscles of the small airways in the lungs and reduces inflammation (swelling and irritation). This gradually eases breathing problems and also helps to prevent asthma attacks.
How Difizma Capsule (Inhalation Powder) Works
Storage
- Hydrate your body: Drink enough water to prevent dehydration and headaches.
- Calm Your Mind: Deep breathing and meditation can help you relax and relieve stress.
- Rest and Recharge: Sleep for 7-8 hours to reduce headache triggers.
- Take rest: lie down in a quiet, dark environment.
- Cold or warm compresses can help reduce tension.
- Stay Upright: Maintain good posture to keep symptoms from getting worse.
- To treat headaches naturally, try acupuncture or massage therapy.
- Over-the-counter pain relievers include acetaminophen and ibuprofen.
- Prescription Assistance: Speak with your doctor about more substantial drug alternatives.
- Severe Headaches: Seek emergency medical assistance for sudden, severe headaches.
- Frequent Headaches: If you get reoccurring headaches, consult your doctor.
- Headaches with Symptoms: Seek medical attention if your headaches include fever, disorientation, or weakness.
- Contact your doctor immediately if you're experiencing a fast heart rate, palpitations, or other heart-related symptoms. This is crucial to determine whether the symptoms are related to your medication.
- Your doctor may need to adjust your medication regimen to alleviate the fast heart rate symptoms. This could involve changing the medication, reducing the dosage, or adding new medications to counteract the side effects.
- Follow your doctor's advice on monitoring your heart rate and blood pressure. This will help track any changes and ensure your heart rate returns normal.
- If you experience severe symptoms such as chest pain, dizziness, or shortness of breath, seek immediate medical attention. These symptoms can indicate a more serious condition that requires prompt treatment.
- Throat pain can worsen if there is no proper rest for your throat.
- Drink plenty of warm fluids and frequently gargle with salt water.
- Humidify the surrounding air using a humidifier, as dry air may increase dryness and throat pain.
- Consider taking lozenges or hard candy throat pain relievers, which give a soothing effect.
- Take enough rest and stay in warm places to reduce pain quickly.
- Tell your doctor about the cough symptoms you're experiencing, which may be triggered by your medication.
- Your doctor may adjust your treatment plan by changing your medication, adding new medications, or providing guidance on managing your cough symptoms.
- Practice good hygiene, including frequent handwashing, avoiding close contact with others, and avoiding sharing utensils or personal items.
- Stay hydrated by drinking plenty of fluids, such as water, tea, or soup, to help thin out mucus and soothe your throat.
- Get plenty of rest and engage in stress-reducing activities to help your body recover. If your cough persists or worsens, consult your doctor for further guidance.
- Get ample rest; this helps your stomach to settle.
- Eat soft and easy-to-digest foods like white rice, bananas, crackers and apple sauce.
- Include probiotics-rich food like yoghurt, kefir, or miso, as they help maintain gut functioning.
- Wash your hands properly before preparing/eating food and after using the toilet.
- Drink water, broth or diluted fruit juice to replace electrolytes and lost fluids.
- Avoid caffeine, alcohol, high-fat, fried and fast foods, as well as sweetened beverages.
What if I have taken an overdose of Difizma Capsule (Inhalation Powder)
Drug Warnings
- If you are allergic to mometasone furoate, indacaterol, glycopyrronium bromide, or any other ingredients of Difizma Capsule (Inhalation Powder) , please tell your doctor.
- Difizma Capsule (Inhalation Powder) is not recommended for children below 18 years.
- If you are pregnant or breastfeeding, take your doctor’s advice before using Difizma Capsule (Inhalation Powder) .
- If you have chest tightness, wheezing, coughing or breathlessness immediately after using Difizma Capsule (Inhalation Powder) , stop using it and consult a doctor right away as these may be signs of unexpected airways tightening.
- If you have eye pain or discomfort, visual haloes (seeing bright circles around lights), temporary blurring of vision, or coloured images in association with red eyes, contact your doctor, as these may be signs of an attack of angle-closure glaucoma.
- If your asthma is not getting better or if it worsens after you have started using Difizma Capsule (Inhalation Powder) , talk to your doctor.
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Co-administration of Difizma Capsule (Inhalation Powder) with Mifepristone may make Difizma Capsule (Inhalation Powder) less effective as a therapy.
How to manage the interaction:
Taking Difizma Capsule (Inhalation Powder) with Mifepristone is generally avoided as it can result in an interaction, it can be taken only when a doctor has advised it. Do not stop using any medications without talking to a doctor.
Taking Pramlintide with Difizma Capsule (Inhalation Powder) can cause slow stomach emptying or slow the intestinal absorption of nutrients.
How to manage the interaction:
Although using Pramlintide and Difizma Capsule (Inhalation Powder) together can lead to an interaction, it can be taken if advised by your doctor.
Coadministration of levodopa with Difizma Capsule (Inhalation Powder) can significantly decrease the blood levels of levodopa.
How to manage the interaction:
Although using Difizma Capsule (Inhalation Powder) and levodopa together can lead to an interaction, it can be taken if advised by your doctor. However, if you experience any unusual symptoms contact the doctor immediately. Do not stop using any medications without a doctor's advice.
Co-administration of Digoxin and Difizma Capsule (Inhalation Powder) may increase the serum concentration of Digoxin and increase the risk or severity of adverse effects.
How to manage the interaction:
Although there is a possible interaction between Digoxin and Difizma Capsule (Inhalation Powder), you can take these medicines together if prescribed by a doctor. However, if you experience sudden dizziness, lightheadedness, fainting, shortness of breath, confusion, loss of appetite, nausea, vomiting, diarrhea, change in vision such as blurry or yellow vision, fatigue, and fast or irregular heartbeat, contact your doctor immediately. Do not discontinue any medications without first consulting your doctor.
The combined use of secretin and Difizma Capsule (Inhalation Powder) can inhibit gastric acid secretion.
How to manage the interaction:
Although using secretin and Difizma Capsule (Inhalation Powder) together can lead to an interaction, it can be taken if advised by your doctor.
The use of Potassium chloride and Difizma Capsule (Inhalation Powder) can increase the irritant effects of potassium on your stomach and upper intestine.
How to manage the interaction:
Using Potassium chloride and Difizma Capsule (Inhalation Powder) together can lead to an interaction, however, it can be taken if advised by a doctor. However, if you experience severe stomach pain, bloating, sudden lightheadedness or dizziness, nausea, vomiting (especially with blood), decreased hunger, or dark, tarry stools, consult the doctor immediately. Do not discontinue any medications without a doctor's advice.
Using propranolol together with Difizma Capsule (Inhalation Powder) may reduce the benefits of both medications, since they have opposing effects in the body. In addition, propranolol can cause breathing problems.
How to manage the interaction:
Although taking Propranolol together with Difizma Capsule (Inhalation Powder) can possibly result in an interaction, they can be taken together if prescribed by your doctor. However, if you experience any unusual symptoms contact your doctor immediately. Do not stop using any medications without first talking to your doctor.
Combining Labetalol and Difizma Capsule (Inhalation Powder) could decrease the medical benefits of both medications.
How to manage the interaction:
Although taking Labetalol and Difizma Capsule (Inhalation Powder) together can result in an interaction, it can be taken if a doctor has prescribed it. Do not forget to inform a doctor, if you have severe chronic obstructive pulmonary disease (COPD) or a history of asthma, as Labetalol is often not advised in these patients. Do not discontinue any medications without a doctor's advice.
Taking Carvedilol and Difizma Capsule (Inhalation Powder) may reduce the treatment outcomes.
How to manage the interaction:
There may be a possibility of interaction between Difizma Capsule (Inhalation Powder) and Carvedilol, but it can be taken if prescribed by a doctor. Do not stop using any medications without a doctor's advice.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Diet & Lifestyle Advise
- Eat a healthy diet and exercise regularly to strengthen your breathing muscles and boost your immune system.
- Avoid foods such as cabbage, beans, garlic, onions, shrimp, pickled food, dried fruits, fried foods, carbonated drinks, wine, bottled lemon, and lime juice, as they may worsen asthma symptoms.
- Do meditation, deep breathing, regular exercise, and try progressive muscle relaxation techniques to get relief from stress and reduce the risk of an asthma attack.
- Quit smoking as it may reduce the effectiveness of the Difizma Capsule (Inhalation Powder) and irritate the lungs, worsening breathing problems.
- Learning breathing exercises will help you move more air in and out of your lungs.
Habit Forming
Therapeutic Class
All Substitutes & Brand Comparisons
Alcohol
Consult your doctor
The interaction of Difizma Capsule (Inhalation Powder) with alcohol is unknown. If you have any concerns, discuss them with your doctor.
Pregnancy
Consult your doctor
Difizma Capsule (Inhalation Powder) should be used by pregnant women only if the doctor thinks the benefits outweigh the risks. Therefore, inform your doctor if you are pregnant before using Difizma Capsule (Inhalation Powder) .
Breast Feeding
Consult your doctor
It is unknown whether Difizma Capsule (Inhalation Powder) is excreted in human milk. Your doctor decides whether to discontinue breastfeeding or to discontinue Difizma Capsule (Inhalation Powder) , taking into account the benefit of breastfeeding for the child and the benefit of therapy for the mother. Therefore, inform your doctor if you are breastfeeding before using Difizma Capsule (Inhalation Powder) .
Driving
Safe if prescribed
Difizma Capsule (Inhalation Powder) usually does not affect your ability to drive or operate machinery.
Liver
Consult your doctor
Consult your doctor before using Difizma Capsule (Inhalation Powder) if you have severe liver problems.
Kidney
Consult your doctor
Consult your doctor before using Difizma Capsule (Inhalation Powder) if you have severe kidney problems.
Children
Unsafe
Difizma Capsule (Inhalation Powder) is not recommended for children and adolescents below 18 years of age.
Heart
Difizma Capsule (Inhalation Powder) should be used cautiously in heart patients due to possible effects on heart rate and rhythm. Always consult your doctor before use, especially if you have arrhythmias or cardiovascular disease.
Geriatrics
Caution
Difizma Capsule (Inhalation Powder) can be used by geriatric individuals, but with caution. Older adults may be more sensitive to side effects like increased heart rate, dry mouth, or throat irritation, so medical supervision is essential.
FAQs
Difizma Capsule (Inhalation Powder) is indicated as a maintenance treatment of asthma in adults. Asthma is a long-term lung disease in which the muscles around the smaller airways become tight (bronchoconstriction) and inflamed, leading to difficulty breathing.
Difizma Capsule (Inhalation Powder) works by relaxing the muscles of the small airways in the lungs and reducing inflammation (swelling and irritation). Thus, it helps ease breathing problems gradually and prevent asthma attacks.
Do not stop using Difizma Capsule (Inhalation Powder) if you feel well. You are advised to use Difizma Capsule (Inhalation Powder) every day and not only when you have breathing problems or other asthma symptoms to ensure it controls your asthma properly.
Difizma Capsule (Inhalation Powder) does not relieve a sudden asthma attack. Therefore, it is advised to always carry a rescue inhaler to treat sudden asthma symptoms.
Difizma Capsule (Inhalation Powder) powder for inhalation is a triple-therapy medication that combines Glycopyrronium, Indacaterol, and Mometasone Furoate. It is used for the maintenance treatment of Chronic Obstructive Pulmonary Disease (COPD) and Asthma, helping to improve lung function, reduce symptoms, and enhance quality of life.
The common side effects of Difizma Capsule (Inhalation Powder) that may occur in some individuals are a runny nose, a sore throat, sudden difficulty breathing, chest tightness with coughing or wheezing, oral thrush (a fungal infection in the mouth), headache, or a fast heartbeat. Most of these side effects do not require medical attention and gradually resolve over time. However, if these side effects persist or worsen, please consult your doctor.
To use inhalation capsules, start by washing your hands thoroughly. Open the capsule package, remove one capsule, and place it into the inhaler device. Close the inhaler and pierce the capsule by twisting or clicking the device. Breathe out fully, then place the mouthpiece into your mouth and seal your lips around it. Inhale deeply and slowly through the mouthpiece, hold your breath for 2-3 seconds, and exhale slowly. Repeat as instructed by your doctor or as indicated on the prescription label. Always follow the instructions provided with your inhaler device, use a new capsule for each dose, and dispose of the used capsule and packaging responsibly.
It is strictly advised not to smoke while using Difizma Capsule (Inhalation Powder) . Difizma Capsule (Inhalation Powder) helps in managing asthma by improving lung function, but smoking makes these benefits useless as smoking can worsen asthma symptoms.
If you have diabetes and are taking Difizma Capsule (Inhalation Powder) , monitor your blood sugar and rinse your mouth after use to prevent infection. If you notice any significant changes in your blood sugar levels, consult your healthcare provider for advice and adjustments to your treatment plan.
Yes, it is essential to continue taking Difizma Capsule (Inhalation Powder) even if you start feeling better and complete the entire treatment course. Stopping too soon can lead to incomplete recovery and the return of symptoms. Instead, report your progress to your doctor and follow their advice. They will guide you on how to safely taper off the medication if necessary, monitor for any potential side effects, and ensure your health is fully cleared.
The recommended dose of Difizma Capsule (Inhalation Powder) for inhalation depends on factors like age, sex, and disease condition. Generally, one capsule is recommended for a day. However, follow your doctor's instructions for optimal results.
If your symptoms persist or fail to improve, consult your doctor. Please note that it may take some time to fully heal your condition, as the treatment process can be gradual. However, if you don't see improvement, your doctor may need to reassess your condition, adjust the dosage, or consider alternative treatments. Don't hesitate to consult your doctor with concerns or questions about your progress.
Country of origin
Manufacturer/Marketer address
Customers Also Bought
Disclaimer
Author Details
We provide you with authentic, trustworthy and relevant information
Buy best Respiratory System products by
Cipla Ltd
Glenmark Pharmaceuticals Ltd
Lupin Ltd
Alkem Laboratories Ltd
Sun Pharmaceutical Industries Ltd
Mankind Pharma Pvt Ltd
Macleods Pharmaceuticals Ltd
Zydus Healthcare Ltd
Leeford Healthcare Ltd
Dr Reddy's Laboratories Ltd
Zydus Cadila
Pristine Pearl Pharma Pvt Ltd
Abbott India Ltd
Intas Pharmaceuticals Ltd
Alembic Pharmaceuticals Ltd
German Remedies Ltd
Centaur Pharmaceuticals Pvt Ltd
Aristo Pharmaceuticals Pvt Ltd
Zuventus Healthcare Ltd
Wockhardt Ltd
Koye Pharmaceuticals Pvt Ltd
Micro Labs Ltd
Ipca Laboratories Ltd
GlaxoSmithKline Pharmaceuticals Ltd
Med Manor Organics Pvt Ltd
Seagull Pharmaceutical Pvt Ltd
Torque Pharmaceuticals Pvt Ltd
Blue Cross Laboratories Pvt Ltd
Medishri Healthcare Pvt Ltd
Yash Pharma Laboratories Pvt Ltd
Capital Pharma
East West Pharma India Pvt Ltd
Fourrts India Laboratories Pvt Ltd
Indiabulls Pharmaceuticals Pvt Ltd
Tablets India Ltd
Uniza Healthcare Llp
Adonis Laboratories Pvt Ltd
Divine Savior Pvt Ltd
FDC Ltd
Shreya Life Sciences Pvt Ltd
Corona Remedies Pvt Ltd
Indoco Remedies Ltd
J B Chemicals & Pharmaceuticals Ltd
Unipark Biotech Pvt Ltd
Vasu Organics Pvt Ltd
Wings Pharmacuticals Pvt Ltd
Apex Laboratories Pvt Ltd
Best Biotech
Biological E Ltd
Skn Organics Pvt Ltd
Wanbury Ltd
Eysys Pharmaceutical Pvt Ltd
Healthgate Pvt Ltd
Icarus Health Care Pvt Ltd
Intra Life Pvt Ltd
Stedman Pharmaceuticals Pvt Ltd
Comed Chemicals Ltd
Entod Pharmaceuticals Ltd
Innoglide Pharmaceuticals Pvt Ltd
Lincoln Pharmaceuticals Ltd
Navil Laboratories Pvt Ltd
Precept Pharma
Stryker Pharma Pvt Ltd
Torrent Pharmaceuticals Ltd
Dolvis Bio Pharma Pvt Ltd
Elder Pharmaceuticals Ltd
Emcee Pharmaceuticals (P) Ltd
Geno Pharmaceuticals Pvt Ltd
La Renon Healthcare Pvt Ltd
Megma Healthcare Pvt Ltd
Pfizer Ltd
Prevego Healthcare & Research Pvt Ltd
Zee Laboratories Ltd
Balin Healthcare Pvt Ltd
Brinton Pharmaceuticals Ltd
Embiotic Laboratories (P) Ltd
Incite Pharmaceuticals
Kepler Healthcare Pvt Ltd
Modi Mundipharma Pvt Ltd
Sanatra Healthcare Ltd
Timon Pharmaceuticals Pvt Ltd
Wellok Pharma
Aar Ess Remedies Pvt Ltd
Bacans Biotech Pvt Ltd
Chemo Healthcare Pvt Ltd
Foregen Healthcare Ltd
Knoll Pharmaceuticals Ltd
RPG Life Sciences Ltd
Rnd Laboratories Pvt Ltd
Silver Cross Medisciences Pvt Ltd
Steris Healthcare
Thuyam Life Pvt Ltd
Votary Laboratories (India) Ltd
Yuventis Pharmaceuticals
Aglowmed Pharmaceuticals Ltd
Alienist Pharmaceutical Pvt Ltd
Alniche Life Sciences Pvt Ltd
Astra Zeneca Pharma India Ltd
Astrum Healthcare Pvt Ltd
Bio Warriors Pharmaceucticals Pvt Ltd







